A combination of magnetic nanoparticles and chemotherapy drugs achieves greater efficacy against cancer cells
01/07/2024
 |
|
The combination of magnetic field nanoparticles and chemotherapy drugs achieves greater efficacy against cancer cells. Image: Journal of Colloid and Interface Science (Elsevier). |
- Researchers at IMDEA Nanociencia have studied the combined effects of different actions against cancer cells.
- They have been studied in breast, uvea, colon, lung, and pancreatic cancer cell lines.
- This study provides comprehensive and valuable information for the development of drug-based cancer therapies combined with magnetic nanoparticles and hyperthermia.
| Tweet |
Madrid, 1st July, 2024. The path to a cure for cancer is not unique, as the disease is an extremely complex process. Multiple factors are involved in the process of effectively eliminating a tumour and therefore, being able to have different strategies against cancer is key in this regard.
In a recent study, researchers from IMDEA Nanoscience, led by Dr. Gorka Salas and Dr. Álvaro Somoza, propose the use of a new type of multicore magnetic nanoparticles, for which hardly any studies have been carried out on their efficacy against cancer in physiological environments. Specifically, their study analyzes the effects of multinucleus nanoparticles on various cancer cell lines.
Multicore nanoparticles are aggregates of smaller nanoparticles. The selection of these multicore nanoparticles has been made based on the fact that they are among the most heated in magnetic hyperthermia processes. In a process of magnetic hyperthermia, the magnetic material – in this case the nanoparticles – is subjected to an alternating magnetic field that raises its temperature in a controlled way. Hypothetically, if these particles were found in the environment of a tumour, they would heat the cells of the tumour above their critical temperature, causing the death of the cancer cells and deactivating the tumour.
The treatment of magnetic hyperthermia is generally considered by experts to be very appropriate because it uses a magnetic field that has very good penetration into tissues. Raising the temperature of the nanoparticles high enough can help cancer cells die. Currently, this type of treatment is in clinical trials in a few hospitals around the world. Therefore, research is key so that alternative treatments can reach patients and be used in all types of tumours.
Combination of therapies
In their work, researchers from the "Magnetic Nanoparticles" and "Nucleic Acids and Nanoparticles in Nanomedicine" research groups at IMDEA Nanoscience have studied the efficacy of two distinct nanoparticle morphologies in reducing the viability of cancer cell lines. The cultures used have been commercial cell lines of various types of cancer: pancreatic, uvea, lung, colon and breast. The use of these cell lines gives scientists anywhere in the world the possibility of comparing the results of different experiments on the same cells, under reproducible conditions.
In addition, the researchers have gone a step further, adding two types of anti-cancer molecules to the surface of the nanoparticles to enhance the effect. On the one hand, a chemotherapy drug has been used and, on the other hand, microRNAs, which are small molecules of ribonucleic acid (RNA), which in this case act as tumor suppressors.
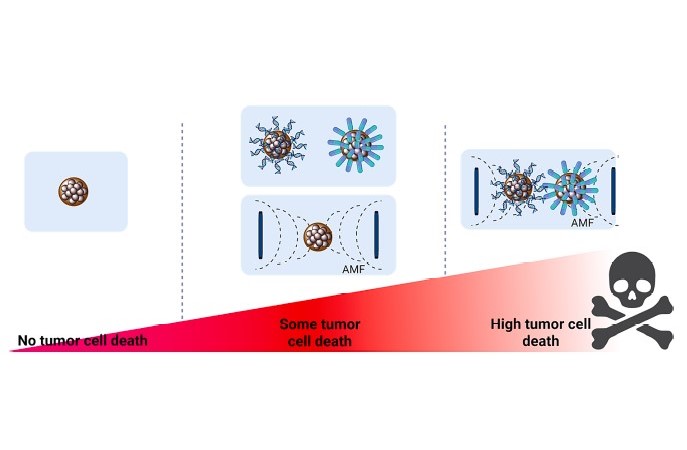
The study of the viability of tumour cells is very exhaustive and all possible combinations have been compared: unmodified nanoparticles; modified by adding either a chemotherapy drug, or by adding microRNAs; or both. And all of the above possibilities have been studied under the effect of hyperthermia heating.
Multicore nanoparticles
A 2012 study showed that multicore nanoparticles – clusters of nanoparticles tightly bound together – were the best among other morphologies for generating heat. It was proven that its specific absorption rate (SAR, watts released per gram of material) was much higher than that of other types of morphologies. Although these results have been around for some time, there are still not many studies on its effectiveness in suppressing cancer cells.
Dr. Gorka Salas' group is now using a new method for synthesizing these multicore nanoparticles, under more favorable conditions than those reported by other research groups. They synthesized two sizes of initial nanoparticles, from which the multicore aggregates, 23 nanometers, and the 'nanoflower' type, 56 nanometers in size, were obtained. They found that the smallest multicore nanoparticles, those of 23 nanometers, heated more quickly. In addition, their coercive field is lower, that is, they are almost superparamagnetic particles that do not magnetize under a magnetic field and do not aggregate with each other. Therefore, they selected this smaller size of multicore nanoparticles for further study.
Functionalization with chemotherapy molecules and microRNA biomolecules
A new function was added to the selected nanoparticles, through their modification with active molecules against cancer. Dr. Álvaro Somoza's group used two different types of molecules: a chemotherapy drug commonly used in the clinic (SN38) and with microRNAs synthesized in Prof. Somoza's own laboratory. MicroRNAs are biomolecules that act by regulating gene expression at the level of messenger RNA. In this case, a cocktail of 4 microRNAs that have shown activity to suppress tumors was prepared.
The binding of these molecules to magnetic nanoparticles is very pertinent. Mainly, the anti-cancer action of the molecules is reinforced by heating by magnetic hyperthermia. In addition, nanoparticles serve as a vehicle to deliver microRNAs where they are convenient, since they are not soluble in water and need a means of transport to the cell. The release of drugs that are linked to nanoparticles is slower, because their half-life is longer, and therefore there is greater control over the process. The dose administered to the patient would also be reduced, reducing side effects after chemotherapy treatment.
En la investigación del Prof. Somoza, la entrega de las moléculas mediante nanopartículas magnéticas fue muy efectiva porque las nanopartículas fueron internalizadas por las células. Se observó un efecto anticancerígeno en todas las líneas celulares, siendo más acusado en algunos tipos de cáncer como el de páncreas o úvea. El efecto de ambos tipos de moléculas fue similar, aunque se encontraron diferencias en páncreas (menor viabilidad de las células con quimioterapia) y pulmón (menor viabilidad de las células con microRNAs).
The effect of hyperthermia
Biological systems are extremely complex networks, in which there are a multitude of variables that can determine the outcome of an experiment. Therefore, collecting as much information as possible from experiments with cells, comparing actions and reproducing evidence, is a very valuable task for designing future cancer treatments.
The heating of the nanoparticles in a hyperthermia treatment depends on the strength of the magnetic field that is applied, and its frequency. The intensity and frequency conditions in the experiments of Dr. Salas' group were chosen taking into account the comfort limit allowed by humans. It was found that the viability of cancer cells was lower when hyperthermia treatment was applied than when it was not. In other words, heating the cells causes fewer cells to survive, 72 hours after treatment. This effect was much more pronounced for pancreatic cancer cells than for other types of cancer, making it clear that cell viability after the application of hyperthermia depends strongly on the cell line considered.
The results showed that the combination of the three therapies, anticancer drugs, gene regulation by microRNAs and magnetic hyperthermia, gave the best results. Cell viability is generally more compromised when the effects of the two therapies are added together. The study, recently published in the Journal of Colloid and Interface Science, exposes all the details of this exhaustive work.
In a disease as complex as cancer, the solutions are therefore also complex. There is no single treatment for a complete cure, but there is the possibility of addressing an effective treatment for each type of tumour and person from different aspects of personalised medicine and nanomedicine. Currently, the application of nanoparticles against cancer is not widespread in clinical practice for several reasons. Intratumoral administration of the nanoparticles is needed, which will remain in the body after treatment; and there are still no studies of its long-term behavior. On the good side, hyperthermia treatment with nanoparticles is a method of "physical action", for which it is more difficult to generate resistance – as is the case with some chemotherapy treatments – and is also transferable to other types of tumours. Cancer treatments based on personalized nanomedicine using nanoparticles are very promising, because they deliver drugs or therapeutic heat directly into cancer cells, having a very precise effect.
The work is a collaboration between researchers from IMDEA Nanoscience, from the research groups "Magnetic nanoparticles" and "Nucleic acids and nanoparticles in nanomedicine". The work has been co-financed by the Severo Ochoa Excellence seal, awarded to IMDEA Nanociencia in 2017 and renewed in 2021.
Reference:
David García-Soriano, Paula Milán-Rois, Nuria Lafuente-Gómez, Ciro Rodríguez-Díaz, Cristina Navío, Álvaro Somoza, Gorka Salas. Multicore iron oxide nanoparticles for magnetic hyperthermia and combination therapy against cancer cells. Journal of Colloid and Interface Science, 670, 73 (2024). DOI: 10.1016/j.jcis.2024.05.046
![]() Publication deposited at IMDEA Nanociencia Repository: https://hdl.handle.net/20.500.12614/3680
Publication deposited at IMDEA Nanociencia Repository: https://hdl.handle.net/20.500.12614/3680
Contact:
Dr. Gorka Salas
https://nanociencia.imdea.org/magnetic-nanoparticles/group-home
www.linkedin.com/in/gorkasalas
Prof. Álvaro Somoza
https://nanociencia.imdea.org/nanobiotechnology/group-home
www.twitter.com/alvarosomoza
Oficina de Divulgación y Comunicación en IMDEA Nanociencia
divulgacion.nanociencia [at]imdea.org
Twitter: @imdea_nano
Facebook: @imdeananociencia
Instagram: @imdeananociencia
Source: IMDEA Nanociencia.