pH-sensitive iridium complexes as catalytic anticancer compounds
 |
11.11.2020
IMDEA Nanociencia researchers present a new family of organometallic iridium complexes that generate cytotoxicity only upon intracellular activation.
Chemotherapy is defined as the use of chemicals to reach and damage cancer cells. On its way towards the tumour, the drugs can affect healthy cells as well. For example, cisplatin, a common drug used in clinical treatments, is not selective and causes unwanted secondary effects such as vomiting, fever, and loss of sensitivity, among others. Such effects often cause to halt the treatment. It is of great importance to find new drugs that can be selectively activated in the tumour for more effective treatments. Understanding the interaction mechanism of new drug candidates within the cell nano-environment is the first step towards reaching the clinic.
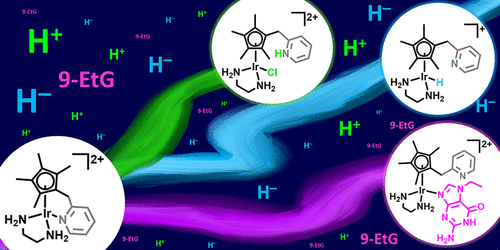
The group of Ana Pizarro at IMDEA Nanociencia investigates the action of iridium complexes as anticancer compounds. These potential metallodrugs can be 100 times more effective than cisplatin, perhaps due to a precise targeting of the cell mitochondria thus reducing the effective dose. The deadly action of iridium complexes is radically different. While cisplatin attacks nuclear DNA, changing its structure and preventing making copies of it, iridium metallodrugs appear to catalise transfer hydrogenation reactions, this is, they facilitate the transfer of hydrogen atoms from some molecules to others, thus breaking the redox balance in the cancer cells and driving them to a certain death.
In their study, published in the ACS journal Inorganic Chemistry, Pizarro’s group synthesised four new iridium complexes stable at pH 7. For two of these complexes, hydrogen transfer inside the cell is proved plausible, as indicated by cytotoxicity experiments in which cells are co-incubated with innocuous amounts of a trigger molecule for the iridium catalytic reaction. Such catalytic reaction appears to compromise cell survival. Furthermore, the authors demonstrate that acidic pH activation is also possible, and efficiently so. The results open up the possibility of designing potent prodrugs activatable in the cancer cell microenvironment, drugs that can exert their action in a catalytic, this is, amplified manner, highlighting the great potential of the “tether design” on iridium(III) half-sandwich compounds.
This work is an outcome of Ana Pizarro’s group at IMDEA Nanociencia and has been partially funded by the 7th Framework Programme through the MEMOTUMCELLMACH project, and the Severo Ochoa Excellence award to IMDEA Nanociencia (2017-2021).
Reference:
Ana C. Carrasco, Vanessa Rodríguez-Fanjul, and Ana M. Pizarro. Activation of the Ir−N(pyridine) bond in half-sandwich tethered iridium(III) complexes. Inorg. Chem. 2020.
DOI: 10.1021/acs.inorgchem.0c02287
Contact:
Ana M. Pizarro
ana.pizarro [at]imdea.org
http://nanociencia.imdea.org/home-en/people/item/ana-maria-pizarro-arranz
Twitter: @pizarroam
IMDEA Nanociencia Outreach Office
This email address is being protected from spambots. You need JavaScript enabled to view it.
+34 91 299 87 12
Twitter: @imdea_nano
Facebook: @imdeananociencia
Instagram: @imdeananociencia
Source: IMDEA Nanociencia